1. Carbonation Overview
In this article, we will briefly discuss the carbonation effect on concrete.
Carbonation is a natural effect that occurs in #concrete at different rates depending on the concentration of carbon dioxide, oxygen, temperature, and water in the area. In the air, we find two main elements composed of carbon, amongst others, and are as follows:
- Carbon monoxide is the fumes from vehicles, small engines, lanterns, grills, and all equipment burning fuel for energy.
- Carbon dioxide is a natural gas emitted from plants, animals, machines burning fossil fuels (coal, natural gas, oil, etc), cement production, volcanos, wildfires, etc.

Carbon monoxide is unreactive and less soluble in water to react with other elements. Therefore, it is less likely to cause carbonation in your concrete. However, carbon dioxide is twice as soluble in water, and when in contact with other elements, it reacts with them.
2. How does carbonation occur?
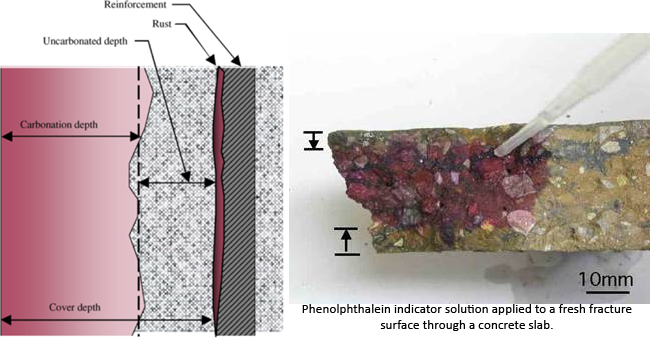
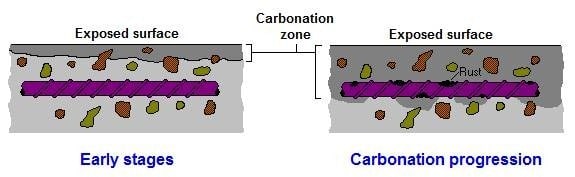
Carbon dioxide enters concrete through the pores, cracks, and capillaries along with oxygen as gases. If moisture is present in concrete, the environment then becomes ideal for the reactions to occur with calcium hydroxide and hydrated calcium silicate in the cement paste to give amounts of calcium carbonate and free water. Calcium carbonates are the black, brittle, dense, and acidic elements that will affect the alkalinity of concrete by lowering it from pH 12.5 down to pH 9 or lower. At that pH concentration, the highly alkaline protective layer around your reinforcing steel, also known as the “Passivation Layer” will degrade to allow the bars to react in the presence of water and oxygen. Temperature is found to have a linear relationship with the carbonation depth. Therefore, it hotter climates, concrete carbonation accelerates.
Reinforcing steel is made by compressing iron ore 6 times and trapping high energy within. When the material starts reacting, it releases that energy and expands to go back to its natural state (Iron Ore). The expansion of the reacted steel will produce a force higher than the tensile strength of the concrete in the cover area, which will cause the latter to crack, and then blow. This is called “Concrete Cancer”.
Carbonation depth is assessed using a solution of phenolphthalein indicator turning pink in contact with alkaline concrete with pH values in excess of 9 and colourless at lower levels of pH. The test is most commonly carried out by spraying the indicator on freshly exposed surfaces of concrete broken from the structure or on split cores. Alternatively, the powder from drill holes can be sprayed or allowed to fall on indicator-impregnated paper.
3. What happens to carbonated concrete?
We are used to hearing that concrete carbonation is very bad as it causes concrete cancer and deterioration. Yes, this is very true! But …

This only happens when using concrete mixes with higher levels of permeable voids, a higher shrinkage rate, a poorly designed structure, and a lack of curing. However, this applies to concrete elements reinforced with traditional black steel.
We need to know that carbonation increases the concrete compressive and tensile strengths thanks to the carbon atoms in the calcium carbonate. It also changes the concrete structure to close the pores making the concrete denser and less permeable.
You can also read Chloride effect on concrete
Photos Credits – ronacrete.co.uk and st-astier.com















2 Comments
To implement preventive measures, carry out vaccination by reacting away the available free lime in the concrete after casting. When there is no free lime in the cover(exposed)area, the risk of carbonation is considerably reduced.
Your writing is a masterclass in subtlety — each word chosen with care, and every sentence a work of art.