1. Chlorine and Chloride
Chloride, a term frequently used in the building sector, refers to the negatively charged version of chlorine, known as the Chloride ion (Cl-). These ions are widely present in the environment (such as air, water, and soil) and play a crucial role in the development and overall well-being of humans, animals, and vegetation.
Nonetheless, when two chlorine atoms come together, they create Chlorine gas, which is harmful.
At the same time, Chlorine and Chloride are processed in industries to manufacture a variety of goods, including PVC, chlorinated solvents, pesticides, polymers, synthetic rubbers, refrigerants, table salts, and concrete enhancers, among others.
Let’s now dive into the world of concrete and explain the effects of chlorine or chloride when in contact with the cement paste in the presence of the activator “Water”.
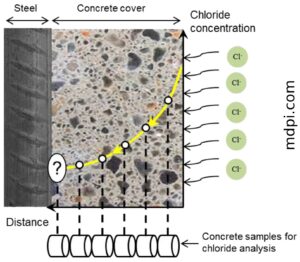
Chloride ions require water molecules in both liquid and gas forms to move around. Then either seeps through or lands on your concrete structure. Here is what will happen if the concrete is not optimised and protected against moisture ingress:
2. Effect of Chloride on concrete
- The salts (Calcium Chloride, which could also be found containing other elements) will react with the free Calcium hydroxide in the cement paste to form a new chemical called Calcium oxychloride (CAOXY) in deposits in the presence of water and oxygen. The salt crystals expand with water creating stresses within the capillaries and microcracks.
When the water evaporates, it then relieves the pressure. The repetitive swelling and shrinking will cause a loss of bond between all the concrete ingredients. In structural terms, the phenomenon will cause a loss of concrete tensile and flexural strengths, hence crumbling.

- If the issue is not addressed at the first signs, the chloride ions will continue their diffusion deep into the concrete to reach the passivation layer protecting your steel. Since Calcium oxychloride is both acidic and basic, the acidity will lower the pH of the concrete from 12.5 down to 9 or even lower.
At this alkalinity level, the passivation layer (Iron Oxide) will lose its efficacity as it’s purely alkaline, hence allowing the Chloride ions to react with the Iron in the presence of water and oxygen to give Hydrated Iron Oxide.
The reaction will release the trapped energy within the steel bars and expand up to 6 times while regaining its natural state (Iron Ore).
This phenomenon is known as Concrete Cancer. This could easily be identified from the straight, raised and short cracking along the closest bars to the surface and spalling. - The Chloride ions could be within the concrete mix itself. The aggregates, the sand, the water, or the admixture used could well contain higher contents of Chloride ions that will eventually affect the durability of the concrete, causing concrete spalling. Other types of internal attacks occur known as Alkali Aggregate Reaction, which could be either Alkali Silica Reaction ASR or Alkali Carbonate Reaction ACR.
Alkali–silica reaction (ASR) is a chemical reaction between alkalis in Portland Cement and certain types of silica in some aggregates. It causes the formation of expansive gels, which causes the concrete to crack. The cycle is repeated until craze cracking occurs on the surface.
Whether you are building a new structure or remediating an existing one, it is important to seek professional advice from someone who understands concrete technology and behaviour, water dynamics, concrete durability, waterproofing, and engineering.
Baleh Consulting can assist.
Written by:
Hacène Baleh
Linkedin Profile
01/08/2024
















2 Comments
Hi there, just wanted to mention, I liked this blog post.
It was practical. Keep on posting!
Everything is very open with a very clear explanation of the challenges.
It was definitely informative. Your site is very useful.
Thank you for sharing!